(LONG BEACH, CA.) – The storage tanks of Rachelle Laboratories, Inc. stand like grain silos along the Terminal Island Freeway in Long Beach, California. They are filled with hundreds of tons of Chlorachel, a Chlortetracycline animal feed additive which Rachelle markets for livestock and poultry.
From the freeway, the Rachelle complex looks like an oil refinery. Iron catwalks, steel piping, stacks emitting steam surround the large fermenters, crystalizers, blenders and baggers. Warehouses are filled with multi-walled 50 pound plastics sacks and hundred-pound fibre drums of Chlortetracycline stacked up in piles like displays at a supermarket.

For people accustomed to thinking of antibiotics in terms of a few cc’s withdrawn from small rubber stoppered bottles for intramuscular injections, the sheer bulk of the antibiotics manufactured by Rachelle (the nation’s second largest manufacturer of Chlortetracycline after American Cyanamid) is overwhelming. As the Rachelle catalogue for Animal Health and Feed Additive Products reminds prospective buyers, minimum orders for most varieties is one ton.
“This is the greatest country in the world in terms of agricultural production,” says Len Zoller, Rachelle Vice-President of the Long Beach plant. “But the way we grow livestock and poultry today, we couldn’t continue to lead the world without broad spectrum antibiotics like Chlortetracycline.”
In 1979, U.S. pharmaceutical manufacturers marketed $243.7 million worth of antibiotics for animal use. Since 1960, the annual consumption rate has risen almost ten-fold. In 1978, the FDA estimated that over six million pounds of antibiotics ended up in animals, much of it in meat producing animals for human consumption.
Since 1949, when anti-microbial feed additives were first discovered to promote weight gain as well as control disease, more than one billion head of cattle have been raised with them. In the U.S., almost all chickens and turkeys, 85 percent of all hogs, 75 percent of all cattle and 50 percent of all sheep are now fed low daily doses of antibiotics for prolonged periods of time.
In the late 1940’s, Dr. Thomas H. Jukes, now Professor in Residence of Medical Physics at the University of California, Berkeley, discovered what he described at the time as, “A unique phenomena – perhaps without precedent in the history of medicine.” Jukes, and his colleague, E.L.R. Stokstad, found that when young chicks were fed a fermented ration containing the micro-organism Streptomyces Aureofaciens, they showed a dramatic increase in weight gain.
The large pharmaceutical companies soon took note of what Jukes began describing as a “new growth factor,” which promised increased gains of up to 20 percent.
By the 1960’s, the poultry, hog and cattle industry had been revolutionized by the new antibiotic feed additives. Farmers and ranchers were able to raise stock in new, efficient factory-like feedlots and confinement systems, which because of crowding and stress would previously have left animals highly susceptible to disease.
Once word got around that these new “wonder drugs” could bring a substantial reduction in disease as well as an increase in profit margin, farmers flocked to get in on the boon. Whereas in 1960, livestock producers still used only slightly over one million pounds (largely as a feed additive), by the early seventies they were buying approximately eight-million pounds a year, almost an eight-fold increase.
With antibiotics, more animals could be raised in less space, on less feed over a shorter period of time and at a lower cost to the consumer.
For many in the drug and livestock industry, the introduction of antibiotics made it seem as if a millenium was near.
But as antibiotics came to be a larger and larger factor in meat production, a number of microbiologists began to worry about the ability of pathogenic (harmful) bacteria to adapt and develop resistance, rendering the newly discovered generation of wonder drugs ineffective. They were particularly concerned over the way these antibiotics in both human and animal applications killed off susceptible bacteria, leaving only those mutants which were resistant.
In itself, this phenomena was alarming. But when in 1959 Japanese researchers showed that resistant bacteria had the capacity to transfer their resistance to other strains of bacteria, many microbiologists became deeply troubled.
One of these men is Dr. Dwight Hirsch, Associate Professor of Microbiology and Chief of the Microbiology Service at the Veterinary Medical Training College at the University of California, Davis.
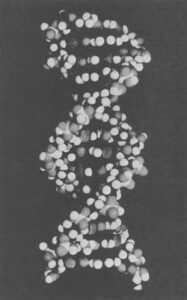
“In effect what we have been doing with the massive use of antibiotics is selecting for resistant mutant strains,” says Hirsch. “But we have also been selecting for bacteria which have recently acquired resistance through a very special mechanism called R-plasmid transfer. The R-factor, or extra chromosomal bits of genetic material (DNA), are genes which are capable of transferring resistance from bacterium to bacterium.
“These R-plasmids are tiny microscopic strands of braided DNA in a double helix shape, which can carry genetic information, like antibiotic resistance. The interesting thing about plasmids, however, is that unlike the regular chromosomes of a cell, they travel from bacterium to bacterium. It doesn’t matter whether a bacteria is pathogenic or non-pathogenic, of animal origin or human origin, these genes can jump independently free from one bacterium to another during the process of conjugation.”
“They’re like two ships at sea transferring mail,” adds Hirsch with a bemused expression.
“These genes have been floating around the environment for years, probably forever. Nobody really knows what their function is in the grand scheme of things. All we know is that by using antibiotics frequently or unnecessarily, we were helping these bacteria with resistant genes along by killing off their natural competition.”
What is extraordinary about the plasmids is not only their almost promiscuous ability to transmit resistance, but the speed at which they do so. One resistant cell can render an entire recipient population of bacteria resistant in a matter of hours. In one experiment, where a patient under observation was given a standard dose of 250mg of tetracycline four times a day, after only 36 hours the percentage of tetracycline resistant E. Coli had risen to 95 percent.
“To me, the implications of this kind of finding are very scary,” continues Hirsch somberly.
“What we are confronted with is the spectre of a completely harmless bacterial strain, like E. Coli, which live in the gut of warm-blooded animals, becoming resistant to a certain antibiotic, and then passing on that resistance to harmful bacteria, which in turn cause diseases such as Salmonella or Gonorrhea in humans.”
Not only have researchers shown the capacity of R-plasmids to transfer resistance to a specific antibiotic, but that the transfer of resistance to one often carries with it resistance to as many as ten other antibiotics at the same time.
Furthermore, Dr. Richard Novick, of the Department of Plasmid Biology, Public Health Research Institute of the City of New York, (one of the path breakers in plasmid research), has estimated that there are at least 356 plasmid-like extrachromosomal elements which can tranfer genetic information from one bacterium into a simple E. Coli. In effect, what he discovered is that these highly mobile and exogenous bits of plasmid DNA are capable of having a greater genetic impact on a recipient, like E. Coli bacterium, than all of the DNA content in the host organism’s own chromosomes.
Perhaps predictably, the pharmaceutical industry is not alarmed about the problems of R-plasmid transfer. I.A. Solomon, Director of Agricultural Research at Pfizer and a spokesman for the Animal Health Institute, (A drug company lobby group in Washington, D.C.) calls R-plasmid transfer “highly improbable” and “theoretical”, claiming that there is “absolutely no evidence that people have gotten sick as a result of subtherapeutic use (of antibiotics) in animals.”
To date, although there have been numerous incidents of death, even epidemics, attributable to resistant forms of disease organisms which have not responded to antibiotic treatment, there has as yet been relatively slim evidence attributing human death to organisms with resistance acquired from animal organisms. When someone becomes ill or dies, it is hard enough to ascertain the presence of resistance, much less trace it back to its origin.
Nonetheless, one case in the United Kingdom does suggest that R-plasmid transfer from animals to humans is not as “improbable” as Mr. Solomon might believe.
During the years 1963-1966, an outbreak of Salmonella swept uncontrollably through cattle herds. Attempts to treat and control the disease were ineffective, and only rendered the pathogen more and more resistant to more and more drugs. As cattle were marketed across Britain, the disease spread. Not only did thousands of cattle die, but many humans became infected. Seven died. Researchers blamed much of their difficulty in treating the disease on plasmid mediated resistance.
A subsequent report presented to Parliament by The Joint Committee on The Use of Antibiotics in Animal Husbandry and Veterinary Medicine, (the Swann Committee report), concluded that there was a hazard to human health caused by the use of antibiotic feed additives. Soon thereafter antibiotics commonly relied upon for human therapy were banned as feed additives, and other subtherapeutic antibiotics required a veterinarian’s prescription.
The Netherlands, Germany, Norway, Sweden and Denmark have also followed suit and similarly restricted the use of antibacterials in animal feed.
“Many farmers are in the habit of just covering themselves with antibiotics”, says Dr. Hirsch. “They’re not treating a specific disease. For instance, they’re just blasting animals with drugs for safekeeping as a prophylaxis before shipping when stress makes them vulnerable to disease. Then further down the line, when the bugs won’t respond to Penicillin at all, you are in real trouble.
“Here at the school of veterinary medicine we teach our students that an antibiotic is a super drug if rationally used. But if it is misused, it will come back to haunt you. It just doesn’t work. In my mind, speaking as a vet, the greatest contributor to the resistance pool has been the food-producing animal.”
“It’s an attack on the free enterprise system, that’s what it is. The next thing you know, they’ll have Dan Rather on T.V. claiming that antibiotics are poisonous,” says Thomas Jukes, who pioneered the field of antibiotic feed additives and is an acknowledged authority in the field, of attempts to restrict animal use of antibiotics. He also recently received the American Chemical Society’s Award for Advancement or Application of Agriculture and Food Chemistry, and has been a consultant to American Cyanamid.
“The usefulness of antibiotic feeding can be measured in the market place,” explains Jukes. “If resistant pathogens appear, the economic advantages will disappear, and so will the use of antibiotic supplementation. In the meantime, I don’t think we have so many cattle, pigs and chickens that we can afford to ignore feeding them by the most economical means.”
“Of course, the minute you call to restrict antibiotic feed additives, the drug and the livestock industry people immediately cry out that the cost of meat will go up,” rejoins Hirsch. “But so what if it goes up a dollar a pound? That doesn’t bother me as much as taking one hell of a risk. It’s a risk which is … Well, I don’t even think you can put a price tag on it.”
“The risk is theoretical,” says D.L.M. Skamser of the American Cyanamid Co., one of the large manufacturers of tetracycline feed mixes. “We need more study.”

The University of California, Davis, Veterinary Medical Teaching Hospital is a large, square, concrete building that rises incongruously above the flat Sacramento Valley.
In front of the hospital several white pick-up trucks, specially outfitted mobile animal hospitals, await calls.
We walk in a side door.
“Putting these things together in one big package is very unsettling”, says Hirsch as we walk. “We’re finding organisms containing genetic information which they shouldn’t contain. They are showing characteristics which belong to other organisms rather than their own. We’re finding resistance generated from organisms which normally inhabit the enteric tract showing up in bacteria from the respiratory tract.
“The Haemophilus influenza, for instance, which causes Meningitis in children, is an interesting example. About five years ago some researchers isolated samples of this bacteria which were resistant to Ampicillin, the drug of choice in treating it. Closer examination showed that these resistance genes were indistinguishable from those we usually find in the gastro-intestinal tract of food-producing animals. They surmised that the resistance factor must have been plasmid mediated and come from a coupling with a completely different strain of bacteria.
“Stanley Falkow, a microbiologist up at Washington University, who is about to move to Stanford, suspects that the Penicillin resistance of the gonococci bacteria (which causes Gonorrhea), may also be able to be passed to the micro-organisms which cause Meningitis in adults.
“Penicillin is such a charm,” says Hirsch nostalgically; as if it were already a thing of the past. “Except for those people allergic to it, it used to be almost foolproof. It’s cheap and non-toxic. If it’s taken away from us by resistance, we’re going to be in trouble. We’ll have to turn to more toxic drugs which often do not perform as well, and can create a whole new host of side effects.
“There has been talk in Washington about limiting the use of Penicillin and Tetracycline, two large animal feed additives, because they are also used commonly for human treatment. That’s all well and good. But we have discovered another ominous aspect of R-plasmid transfer. We have learned that one of the unique features about plasmids is that they link many kinds of resistance. Let’s say that subtherapeutic doses of Tetracycline via animal feed additives had selected for a Tetracycline resistant strain of bacteria. Now, when R-plasmids from this strain transfer resistance to another kind of bacteria, they often not only render it resistant to Tetracycline, but to several other antibiotics such as Streptomycin and Sulfanomids as well. The antibiotics don’t even have to be closely related. We find plasmids which can carry resistance to as many as ten different drugs at the same time.
“You just can’t say, as the animal health industry does, ‘Let’s preserve human drugs for humans and animal drugs for animals’, because these drugs are all linked together. They are linked in the strands of DNA. Resistance to Tetracycline, Streptomycin and Sulfanomids are all located on one piece of DNA. It’s a package proposition: the other varieties of resistance just tag along like a caboose. It’s very difficult to find an antibacterial which only creates a resistance to itself.”
“Recently, we did a survey of normal household dogs here in Yolo County, California. These were animals which, as far as anyone knew, had had no direct contact with antibiotics. And yet over 80 percent of these animals showed up with antibiotic resistant R-plasmids in their gastrointestinal tract.
“Now, the problem vis-a-vis dogs carrying such high levels of resistance is that people are in close contact with them. They pat them, play with them, dogs lick people, and pass on the bacteria from which the R-plasmids can migrate.
“Where are these bugs coming from? That’s the troublesome question. The reason we did this study on dogs was simply to show how widespread these plasmids are.”
Other studies corroborate Hirsch’s fears that animals close to us are capable of transferring resistance to humans. In a 1971 survey, less than 20 percent of wild animals tested (animals which theoretically had no contact with antibiotics) showed any multiple R-factor resistance. Out of the cross section of domestic animals surveyed, 61 percent showed evidence of R-factor resistance.
“We have accomplished in half a century evolutionary changes that couldn’t have happened in a million years”, writes Dr. Ephraim S. Anderson, former Director of the Enteric Reference Laboratory in London, echoing Hirsch’s words. “Recombinant DNA is tiddlywinks compared with what has been done ecologically to bacteria with the emergence of antibacterials.”
Over the past ten years numerous efforts and false starts have been made by the FDA in an attempt to regulate antibiotic animal feed additives. While many independent scientists have strenuously urged that regulatory measures be taken, consultants and lobbyists for the drug industry have equally as strenuously opposed them. Each side has massed expertise and given hundreds of hours of testimony. The sum total of these herculean efforts has been an almost complete stand-off. In the absence of irrefutable evidence, (what some regulation advocates call, “the need to have the corpses piled up before they move”), the FDA, in whose powers the restriction of antibiotics lies, has been indecisive.
The most recent attempt hinged on a report in preparation by the National Academy of Sciences. When it finally came out last spring, it was deeply disappointing to people like Dwight Hirsch.
It concluded that, “Postulations concerning the hazards to human health that might result from the addition of subtherapeutic antimicrobials to feed have neither been proven or disproven… The research necessary to establish a definite risk has not been conducted, and indeed may not be possible… A comprehensive, all encompassing study could not be realized or even approximated because of insurmountable technical difficulties.”
The report also seemed to say that the most logical criteria for regulatory action was whether or not an increased number of people had become ill because of resistance generated by antibiotic feed additives.
“There are no data linking human illness with the subtherapeutic use of antimicrobials in any aspect of animal husbandry”, the report continued, then acknowledging, almost as a footnote, that it was still possible that hazards existed.
“I read that report and found it just incredible”, says Hirsch emphatically. “They seemed to be asking the scientific community to show that antibiotic use in animals was contributing to infectious diseases in humans before they would recommend action. But that, in fact, is not the proof of whether our bacterial pool is getting resistant.
“If you’re going to get sick with a particular micro-organism, you get sick whether or not it is resistant or not. The problem arises not in the numbers of people that contract disease, but in whether or not they can be successfully treated. Obviously, if you’re dealing with resistant bacteria, it will be harder to treat. But there is no connection between the problem of resistance and sensitivity to a drug, on the one hand, and whether or not an organism can cause a disease on the other.”
“As over 100 billion head of livestock of various species have been fed various antibacterials over the past 25 years without one single incident of drug resistant zoonotic disease migrating into human population and creating an uncontrollable epidemic, it is obvious that the probability of such a hideous incident is below 100 billion to one,” says Dr. Robert H. White-Stevens, Bureau of Conservation and Environmental Science, Cook College, Rutgers University to a 1978 Maryland Nutrition Conference.
“It cannot scientifically be concluded that such a fearful event will not possibly occur, but it can be concluded that the probability of such an Andromeda strain (endangered by continued use of antibiotic feed additives) evolving and cutting loose among the public is improbable and so remote to be insignificant and trivial.”
“If this ever goes through,” says Gordon Kempe, Director of Animal Industry Research and Development at American Cyanamid, speaking of proposed controls on subtherapeutic uses of antibiotics, “it will be the largest single mistake the FDA ever made.” He also notes that by the FDA’s own estimates, restriction of animal antibiotics could cost the consumer over a billion dollars.
“There is obviously a tremendous amount of money involved in the manufacture of these feed additives.” says Hirsch with a sigh, when I read him some drug company rebuttals to his conviction that the continued and widespread use of antibiotics is dangerous.
“I hate to think that such consideration might have an influence on the views of any reputable scientists. But I just can’t see how any microbiologist whether in the National Academy or not, can miss the danger.
“The problem of resistance and its transfer has already been demonstrated. Now the FDA wants us to spend millions more of the taxpayers’ dollars to do it over.”
“The pharmaceutical industry will challenge every statement we make”, says Rosa Gryder in the FDA’s Bureau of Veterinary Medicine. “They will say that there is no transferral or resistance, no human health hazard, no degeneration of the genetic pool because of anitbiotics. They will challenge our scientists and our administrators. In the end, we lose most of our cases on administrative and legal slip-ups, on technicalities. They have smart lawyers. Ultimately they can always drag a decision out in court.
“I don’t like making changes in the general food supply without knowing what the consequences are,” continues Gryder. “I guess I’m just conservative that way. Others believe that a little pinch of something else there won’t hurt you. But I must tell you that I have cut down on my meat eating since coming here to the Bureau of Veterinary Medicine.”
“Here we are”, says Hirsch. “We’re going into 1981. They had hearings four or five years ago, and we’ve gotten nowhere. We’re just in a holding pattern. In fact, things are getting worse. The bugs are getting more and more resistant. We’re finding more and more isolates from humans and animals showing resistant organisms.”
As Chief of the Microbiology Service at the U.C. Veterinary Medicine Teaching Hospital at Davis, Dwight Hirsch is in a critical position to follow developments in antibiotic resistance. This hospital cultures hundreds of animal bacteriological samples every week, enabling him to keep an eye on the state of resistance in California’s animal population.
“I wish that those who were playing down the problem of resistance were in our diagnostic laboratory and could see the nature of the strains which come”, says Hirsch wearily.
“When American Cyanamid says there is no evidence that the use of antibiotics fed to animals has led to problems of resistance, and when they say that there is no evidence that this will come to be a problem in the future, they’re… Well, what can you say, except that from a scientific standpoint they are incorrect.
“If we keep using antibiotics, we’re going to create an environment where only those micro-organisms which are resistant to drugs are going to survive. And because of R-plasmids, these resistance genes are going to go wherever they want, into any species of microbe they want, and stay there.
“It’s just a matter of time. It seems to me there is only one solution: cut back on drugs.”
©1980 Orville Schell
Orville Schell is investigating the “Reliance on Drugs and Chemicals in the U.S. Meat Industry.”