Jyoti Madhusoodanan
- 2023

Fellowship Title:
- Human Experiments: Unblinding Clinical Trials
Fellowship Year:
- 2023
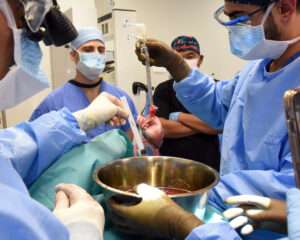
The Allure and Dangers of Experimenting With Brain-Dead Bodies
For scientists who perform medical research on the recently deceased, there are few regulatory or ethical guardrails. This article, written by Jyoti Madhusoodanan, is based on her 2023 Alicia Patterson Foundation fellowship research on human experiments and greater openness behind clinical trials. It appeared in the March 25, 2024 issue of “Undark” a digital magazine funded through the Knight Science Journalism Fellowship Program at MIT. When the Philadelphia-based company Bioquark announced a plan in 2016 to regenerate neurons in brain-dead people, their proposal elicited skepticism and backlash. Researchers questioned the scientific merits of the planned study, which sought to inject stem cells and other materials into recently deceased subjects. Ethicists said it bordered on quackery and would exploit grieving families. Bioquark has since folded. But quietly, a physician who was involved in the controversial proposal, Himanshu Bansal, has continued the research. Bansal recently told Undark that he has been conducting work funded by him and his research team at a private hospital in Rudrapur, India, experimenting mostly with young adults who have succumbed to traffic

How a controversial US drug policy could be harming cancer patients worldwide
Illustration by Karol Banach In August 2021, Amol Akhade, an oncologist at Nair Medical Hospital in Mumbai, India, received an e-mail from the Swiss drug manufacturer Roche recommending the use of a drug named atezolizumab to treat a specific kind of breast cancer. Akhade was surprised. That month, Roche had withdrawn the drug for this purpose in the United States (although it is still approved to treat other kinds of cancer). For the type of breast cancer in question — known as triple-negative because it lacks three key protein markers — atezolizumab was made available in 2019 through the US Food and Drug Administration’s (FDA’s) Accelerated Approval Program. Accelerated approval is a fast-track process designed to place desperately needed drugs in the hands of patients quicker than is possible with conventional drug approval. But a follow-up study found that atezolizumab made little difference to tumour growth, and that people who received it were less likely to survive up to two years after treatment than were those not taking it 1 . When the FDA received
